Quantum Mechanics | Schrödinger's discovery of the Shape of Atoms
I think it could be useful if I restate the central message of the video here...
For clarity:
The shape of hydrogen (and all atoms) is made up by the way the electron spreads itself, as a wave function. This wave function is a blobby shape, and often looks like spheres and donuts put next to one another. The wave function is the electron itself, in a sense. When you don't disturb it, the electron looks like that. When you do disturb it, it becomes a single hard point, and the blobby shape vanishes.
Specific notes and clarifications:
Schrödinger didn't really emphasize the shape of atoms in his discovery, instead everyone considered the big discoveries to be:
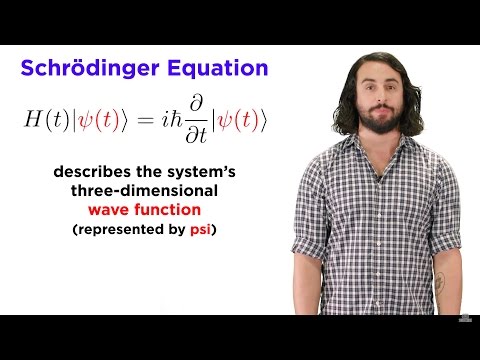
1) The Schrödinger equation
2) The total hydrogen wave function
3) The energy levels of hydrogen
But the shape of hydrogen follows immediately from the total hydrogen wave function, and since we want to look at those shapes, I figured I should focus on them in this vid.
At 1:40 I talk about the planetary model of the atom. There were actually two variations of the planetary model, the Rutherford model and the Bohr model. It was the Bohr model that made these 'very nice predictions' I mention, it gave a relation for the energy levels of hydrogen. It couldn't explain where these energy levels were coming from though, it took Schrödinger's discovery of the total hydrogen wave function to explain their origin.
There were also some other atomic models before the planetary model, for instance the cubic model, the Saturnian model and the plum-pudding model. They are now relevant only in a historic sense.
At 2:03 I simplify the discovery of wave-particle duality in electrons a bit. De Broglie was indeed the first to propose it for electrons, but he was building on previous work by Einstein. Einstein had made a formal definition of wave-particle duality in photons (light), and De Broglie was extending it to matter.
The four situations I list are also more of a hindsight-view that justify De Broglie's pitch. They are: Compton scattering between electrons and photons, the photographic-plate part of the double slit experiment, the crystal-grate part of the double slit experiment and electron free-particle behavior.
At 4:13, I draw eight orbitals of hydrogen as an example, but there are more. Strictly speaking there's an infinite amount of orbitals, of which about the first 80 are important for chemistry and physics. I picked these eight to draw simply because they make nice examples of which shapes hydrogen can take.
Many of those 80 orbitals actually look rather alike. Often you'll have several orbitals that have the same shape, just flipped 90°, or with an additional set of small blobs nested within the big blobs.
The spotty picture I draw at 5:38 of the thousand positions of the electron is somewhat simplified. I draw every position inside the three blobs -- but this is not quite correct. The blobs are what are known as "90%-probability surfaces". Basically, you have a 90% chance of finding the electron within these blobs. The remaining 10% of sightings will fall somewhat outside the blobs. Like any wave, the electron wave function decays slowly and stretches out for quite a while. I didn't want to draw these extra 10%, because I thought it would be confusing.
The argument still holds though: There really is an area in between the blobs where you cannot encounter the electron, called a nodal plane.
At 5:44 I refer to the electron's wave function as 'probability wave function'. This is a slip of the tongue on my part, the phrase is either 'probability distribution' or 'wave function'.
There is also a subtle difference between those two phrases, the probability distribution is the absolute squared of the wave function: P = |Ψ|². But, for the purpose of the video, they are both 'blobby shapes'.
The '40 years of heated debate' I mention at 6:09 was about the interpretation of quantum mechanics, and the philosophical implications. Things like teleportation, determinism and statistical randomness were discussed, leading to several different interpretations, the main ones of which were: The Copenhagen interpretation, the Many Worlds interpretation and Realism.
Einstein (who favored Realism) strongly disliked the statistical, random nature of the wave function, and he summed it up in a famous statement: "I, for one, am convinced that God does not throw dice".
His stance was ultimately disproven in a series of experiments that proved Bell's theorem.